What is a fuel cell?
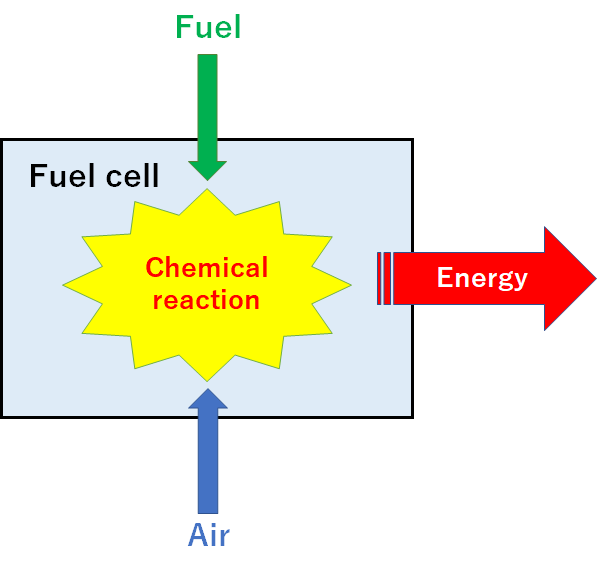
- A fuel cell generates electricity through chemical reactions between a
fuel (often hydrogen) and oxygen from the air. - Fuel cells serve as power generators that can produce electricity
continuously for as long as fuel and oxygen are supplied.
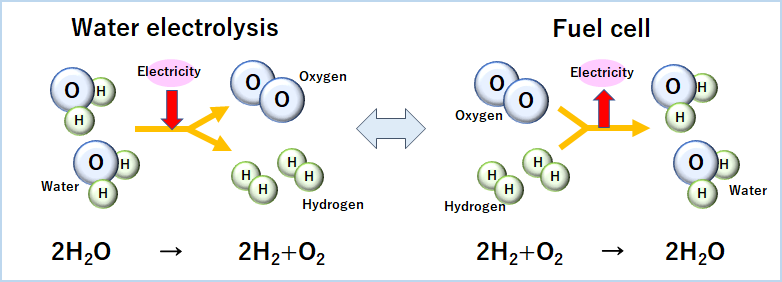
How to generate electricity – the reverse of “water electrolysis”
Water electrolysis: the process whereby water is decomposed into hydrogen and oxygen through the application of electrical energy
↑
↓
Fuel cell: a device that generates electricity through electrochemical reactions between hydrogen and oxygen
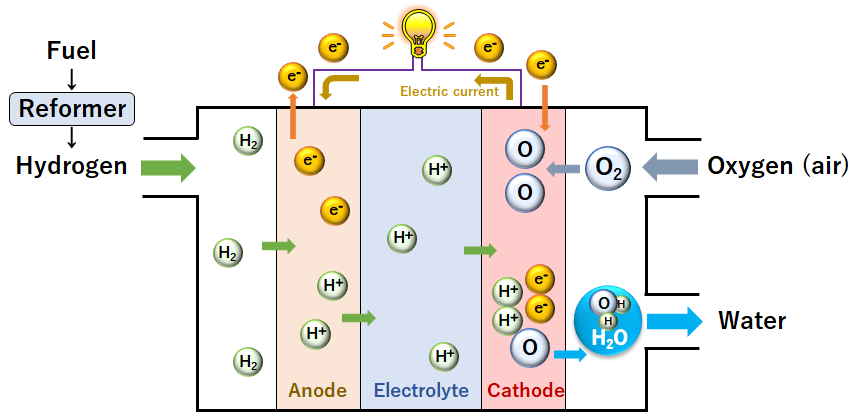
◆Oxygen (O2) in the air is used.
◆Hydrogen (H2) is extracted from natural gas.
How a fuel cell works
- Hydrogen is divided into electrons and hydrogen ions at the anode.
- Electrons produced from the decomposition of hydrogen travel through an external circuit to generate electricity.
- Hydrogen ions then travel through the electrolyte to the cathode where they combine with O2 and electrons supplied from
the external circuit to form water.
References
- Ikeda, Hironosuke. Nenryo Denchi no Subete (All about Fuel Cells). Tokyo, Japan: Nippon Jitsugyo Publishing.
- Homma, Takuya. Zukai, Nenryo Denchi no Subete (All about Fuel Cells, Illustrated). Tokyo, Japan: Kogyo
Chosakai Publishing Co., Ltd. - Website of New Energy and Industrial Technology Development Organization
- Website of the Japan Gas Association
- Website of Tokyo Gas Co., Ltd.
