Global Collaborative Research and Education Center for Integrated Flow Science (IFS-GCORE)
Reactive Flow Systems Laboratory

ProfessorHisashi Nakamura

Assistant ProfessorKeisuke Kanayama
Energy conversion by combustion is used in various energy systems for propulsion, power generation, and heating due to its good load-following capability and high output per size and weight. On the other hand, significant challenges are required to change the fuel from fossils to renewable sources in order to eliminate CO2 emissions. In our laboratory, we are elucidating and modeling the chemical reactions and flame characteristics of new fuels in the renewable energy era through experiments using micro-flow reactors and burners and numerical simulations of the elementary processes, thereby establishing a platform for numerical combustion simulations necessary for the design and development of zero-emission combustors. We are also investigating the effects of combustion with new fuels on combustor walls and materials to be heated. We are also studying the combustion of electrolytes and refrigerants, which are key combustible components, and flame retardants that can be added to them, in order to mitigate fire accidents in batteries that store renewable energy as electricity and heat pumps that provide high-efficiency heating and cooling.
Ammonia combustion – the ultimate environmentally friendly combustion, emitting only nitrogen and water
Ammonia is a promising energy carrier for the future because it can be produced from renewable energy sources, has excellent storage and transport characteristics, and emits no CO2 when used for energy. On the other hand, ammonia is less reactive than hydrocarbons and generates NOx formation from the nitrogen atom in ammonia. Therefore, a new combustor based on fundamental knowledge is needed to achieve high efficiency and low NOx combustion. For design and development, we are investigating the flame characteristics and elementary reaction processes of ammonia, and performing model development and validation.
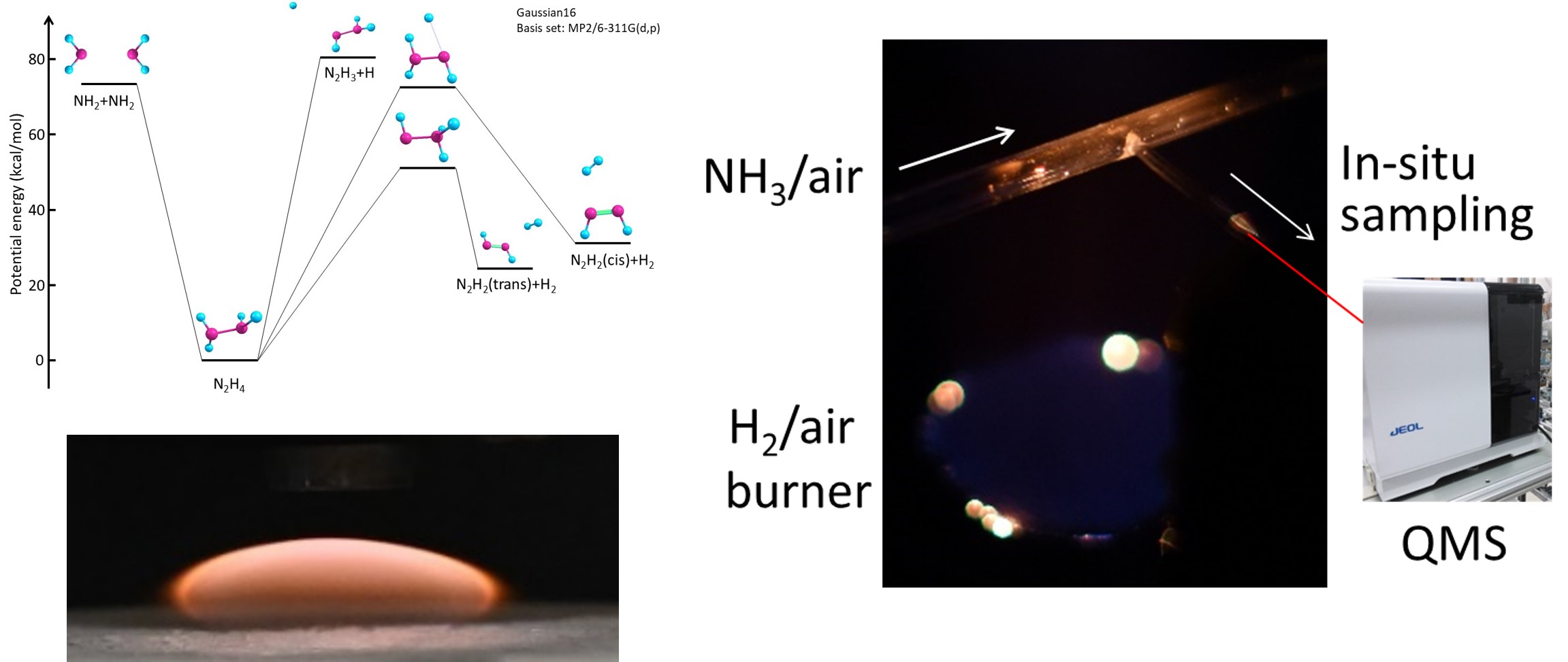 Fundamental combustion experiments (lower left: counterflow flame, right: micro-flow reactor) and quantum chemical computations (upper left) for ammonia
Fundamental combustion experiments (lower left: counterflow flame, right: micro-flow reactor) and quantum chemical computations (upper left) for ammonia
Development of surrogate combustion reaction model of battery electrolytes and flame retardants soluble in them
Since power density and energy density of lithium-ion batteries (LIB) are increasing and opportunities for their use are expanding, mitigation of LIB fire is an important technical issue. For carbonate esters, main components of LIB electrolytes, we are conducting reactivity evaluation and development of combustion reaction model. In addition to carbonate esters, fluoride and phosphorus compounds, which are considered as flame retardants soluble in LIB electrolytes, are also covered. As a result, we are developing a platform for predicting the reactivity of LIB electrolytes and flame retardants soluble in them.
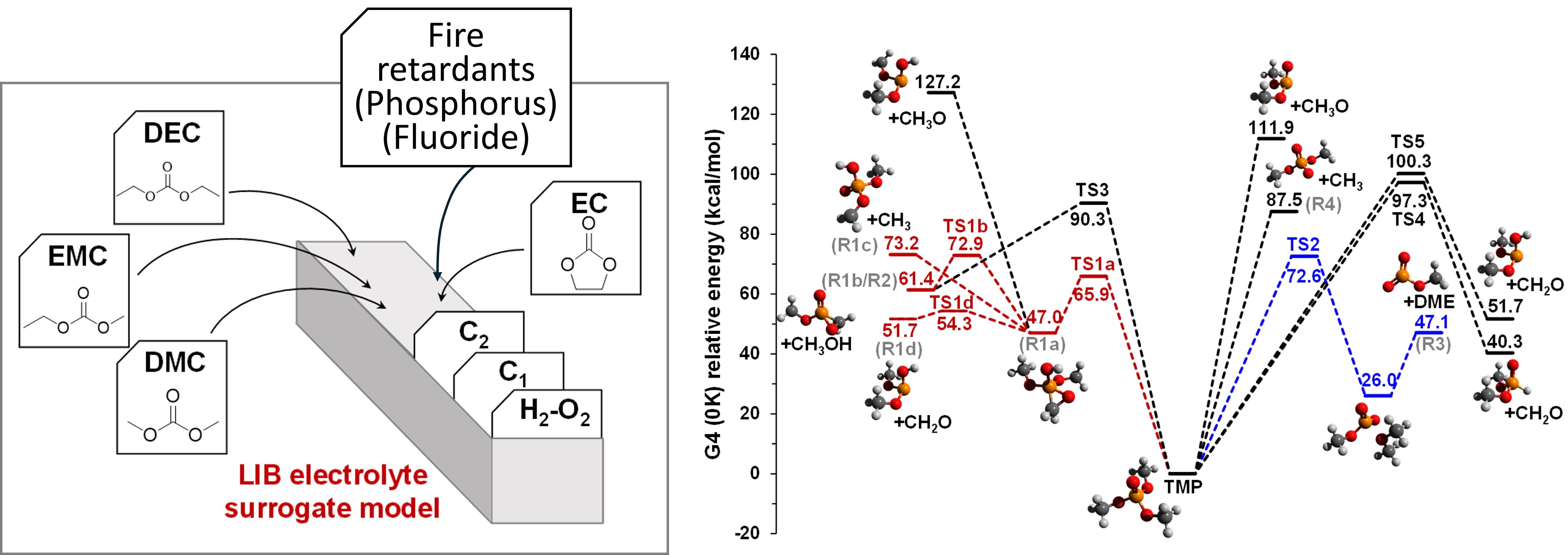 Schematic of LIB electrolyte surrogate combustion reaction model and pyrolysis reaction pathway of a fire retardant
Schematic of LIB electrolyte surrogate combustion reaction model and pyrolysis reaction pathway of a fire retardant
Machine learning-assisted method for generating simplified reaction models
Detailed reaction models include hundreds to thousands of chemical species. Combustion numerical simulations that solve the conservation of all of these species require a huge computational cost. In order to conduct the design and development of combustors and fire simulations with a realistic computational cost, we are developing a method for generating simplified reaction modes that include a smaller number of species while keeping prediction performance of combustion properties. Since combustion phenomena are highly nonlinear, we are particularly developing machine learning-assisted methods to achieve ultra-small reaction models, which are difficult to achieve using conventional methods.
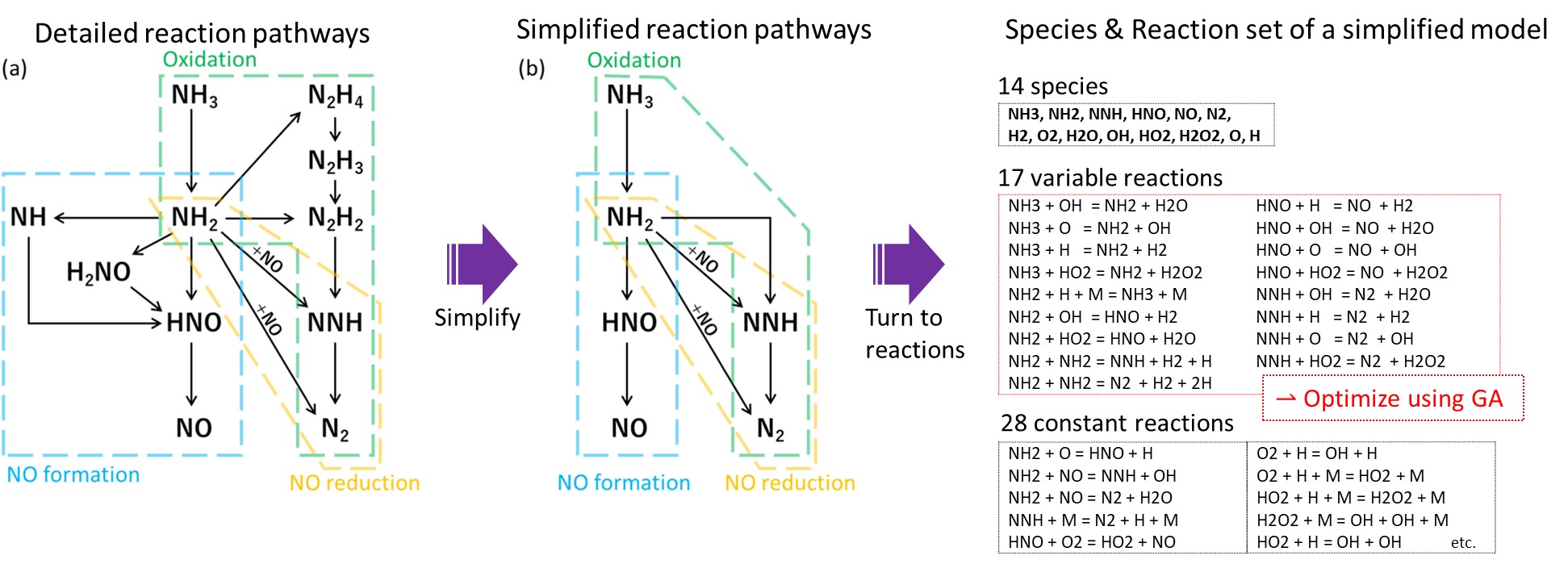 A method for generating a simplified ammonia reaction model using genetic algorithm
A method for generating a simplified ammonia reaction model using genetic algorithm
